KARACALM™
Clinically Tested & Patent-Pending Herbal Sleep & Stress Support Formula–Produces Results Beginning in Just 14 Days*
Produces noticeable improvements in sleep & overall wellbeing within 14 days*
Increases duration of sleep*
Improves ability to fall asleep faster*
Improves deeper sleep*
Improves uninterrupted sleep*
Reduces sleep awakenings*
Reduces feelings of stress*
Decreases serum cortisol*
Reduces hs-CRP*
*These statements have not been evaluated by the Food and Drug Administration. This product is not intended to diagnose, treat, cure, or prevent any disease.
Disclaimer: This website summarizes the results of a randomized, double-blind, parallel and placebo-controlled study. KaraCalm™ is not offered for sale to consumers. The information contained herein is only intended for use by manufacturers for product development purposes and should be reviewed by their legal counsel before the information is used on labels, in marketing or promotional materials or as support for any claim made in connection with a product that contains KaraCalm™.
KaraCalm’s Scientifically Validated Benefits
At the end of the clinical study, the placebo group experienced no statistically significant change, but the KaraCalm group reported...
1 – IMPROVEMENT IN SLEEP*
39% increase (2 hours 15 minute) in total sleep time*
70% decrease (18 minutes to 5 minutes) in onset latency (time taken to fall asleep)*
42% decrease in Wake After Sleep Onset Minutes (duration subject is awake in the night after first falling asleep)*
53% decrease in number of awakenings after first falling asleep*
10% increase (77% to 87%) in sleep efficiency*
2 – REDUCTION IN STRESS*
40% decrease in PSS (Perceived Stress Score)*
30% decrease in Serum Cortisol*
64% decrease in hs-CRP (High Sensitivity C-Reactive Protein)*
THE CLINICAL STUDY
-

OBJECTIVE
To study KaraCalm’s ability to improve sleep and manage stress
-

PARTICIPANTS
60 healthy male and female subjects ranging from 18 to 54 years old, split evenly between two groups
-

METHOD
A randomized, double-blind, parallel, placebo-controlled study with measurments at Day 2, 14 and 56
-

DAILY DOSAGE
500 mg of KaraCalm for one group and 500 mg placebo for the other group
Scientifically Proven Benefits for Improved Sleep*
39% Increase in Total Sleep Time (2 hours 15 minutes) vs No Statistically Significant Change for the Placebo Group*
TOTAL SLEEP TIME
By Day 14, KaraCalm subjects experienced a 21% increase in total sleep time vs a 2% non-statistically significant increase in the placebo group.*
By Day 56, KaraCalm subjects experienced a 39% increase in total sleep vs a 6% non-statistically significant decrease in the placebo group.*
Measured by the Actigraphy watch.
70% Decrease (18 to 5 minutes) in Onset Latency vs No Statistically Significant Change for the Placebo Group*
ONSET LATENCY
By Day 14, KaraCalm subjects experienced a 43% decrease in onset latency vs a 3% non-statistically significant decrease in the placebo group.*
By Day 56, KaraCalm subjects experienced a 70% decrease in onset latency vs a 1% non-statistically significant decrease in the placebo group.*
Time taken to fall asleep once the subject decides to go to sleep - measured by the Actigraphy watch.
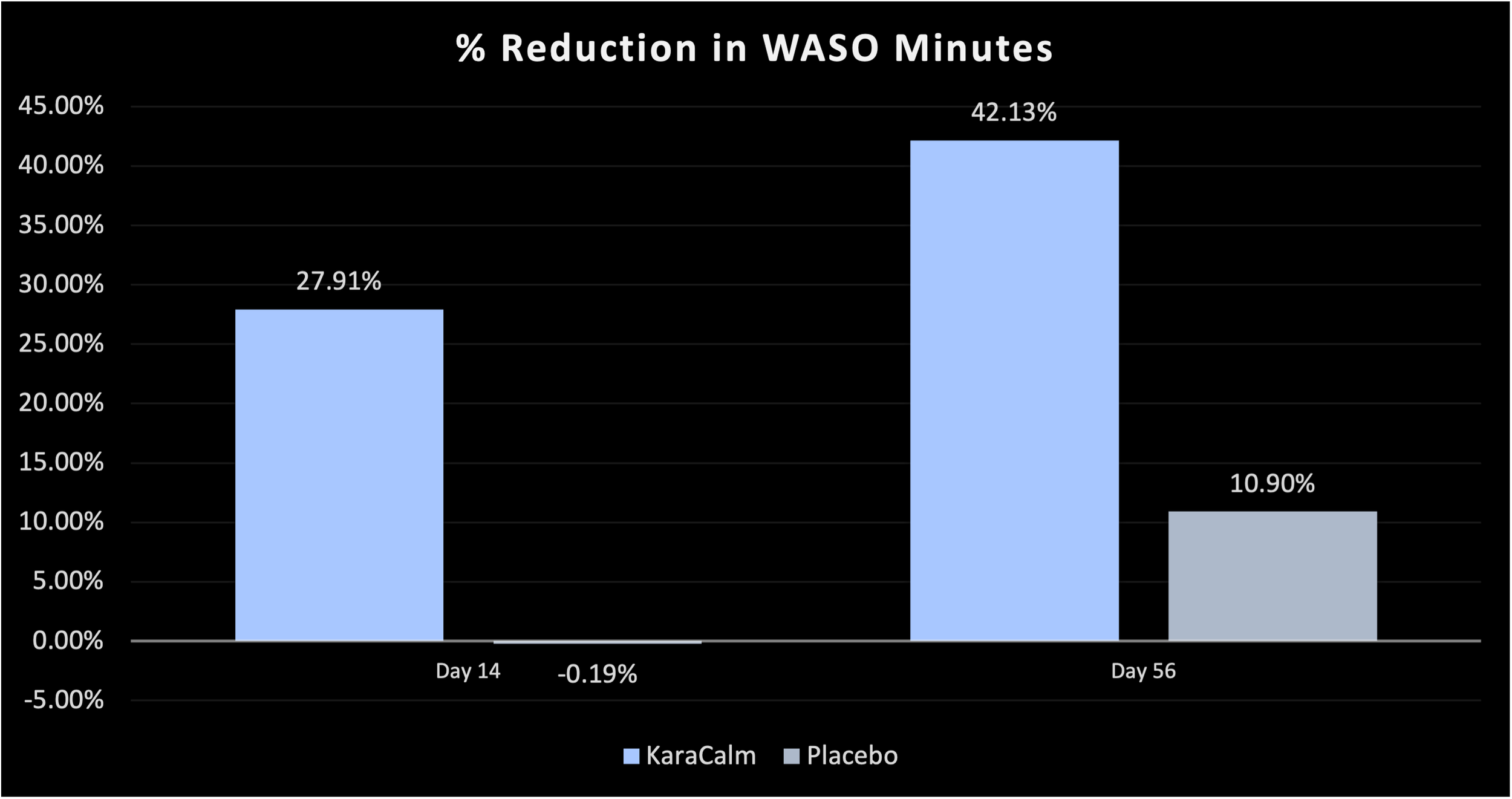
42% Decrease in WASO Minutes vs No Statistically Significant Change for the Placebo Group*
WASO MINUTES (Wake After Sleep Onset)
By Day 14, KaraCalm subjects experienced a 28% decrease in WASO minutes vs a negligible change in the placebo group.*
By Day 56, KaraCalm subjects experienced a 42% decrease in WASO minutes vs an 11% non-statistically significant decrease in the placebo group.*
Number of minutes a person is awake after first falling asleep - measured by the Actigraphy watch.
53% Decrease in # of Awakenings After First Falling Asleep vs No Statistically Significant Change for the Placebo Group*
NUMBER OF AWAKENINGS
By Day 14, KaraCalm subjects experienced a 29% decrease in # of awakenings vs a 2% non-statistically significant decrease in the placebo group.*
By Day 56, KaraCalm subjects experienced a 53% decrease in # of awakenings vs a 1% non-statistically significant decrease in the placebo group.*
Number of times subject is awake in the night - measured by the Actigraphy watch. Awakenings are not just conscious awakenings, but also awakenings the subject is not aware of but is captured by the Actigraphy watch.
10% Increase (77% to 87%) in Sleep Efficiency vs No Statistically Significant Change for the Placebo Group*
SLEEP EFFICIENCY
By Day 14, KaraCalm subjects saw an increase in sleep efficiency from 77% to 82% vs a non-statistically significant 76% to 77% increase in the placebo group.*
By Day 56, KaraCalm subjects saw an increase in sleep efficiency from 77% to 87% vs a non-statistically significant 76% to 78% increase in the placebo group.
The ratio between time a person spends asleep & total time dedicated to sleep. A sleep efficiency of 85%+ is considered optimal.
Scientifically Proven Benefits for Stress Reduction*
40% Decrease in PSS (Perceived Stress Score) vs No Statistically Significant Change for the Placebo Group*
PERCEIVED STRESS SCORE (PSS)
By Day 14, KaraCalm subjects experienced a 14% decrease in stress vs a negligible change in the placebo group.*
By Day 56, KaraCalm subjects experienced a 40% decrease in stress vs a 6% non-statistically significant decrease in the placebo group.*
A measure of how much stress subjects experience, ranging from 0 to 40. Scores 0-13 considered low stress, 14-26 moderate stress and 27-40 high stress.
30% Decrease in Serum Cortisol vs No Statistically Significant Change for the Placebo Group*
SERUM CORTISOL
By Day 28, KaraCalm subjects experienced a 19% decrease in cortisol levels vs a non-statistically significant 2% decrease in the placebo group.*
By Day 56, KaraCalm subjects experienced a 30% decrease in cortisol levels vs a non-statistically significant 2% decrease in the placebo group.*
Lower cortisol levels indicate a reduction in stress.
64% Decrease in hs-CRP (High Sensitivity C-Reactive Protein) vs No Statistically Significant Change for the Placebo Group*
HIGH SENSITIVITY C-REACTIVE PROTEIN (hs-CRP)
By Day 28, KaraCalm subjects experienced a 13% decrease in hs-CRP levels vs a non-statistically significant 1% decrease in the placebo group.*
By Day 56, KaraCalm subjects experienced a 64% decrease in hs-CRP levels vs a non-statistically significant 3% decrease in the placebo group.*
A reduction in hs-CRP indicates a reduction in overall stress.
THE HERBAL FORMULA
KaraCalm is a blend of these herbal extract ingredients with a specific unique blending ratio and standardized to specific active compounds:
-

Valeriana Officinalis
-

Passiflora Incarnata
-

Ocimum Sanctum
-

Ziziphus Jujuba
-

Rosmarinus Officinalis
-

Nigella Sativa
OUR QUALITY STANDARDS
Karallief® has successfully united the powerful forces of nature, science and innovation to develop our breakthrough KaraCalm formula.
Our company is unlike many ingredient suppliers that use inferior, untested materials that lack quality control measures. Instead, we pride ourselves on offering safe, efficacious and scientifically backed ingredients that are tested through thorough quality control procedures using high-end testing equipment.
Rigorous Quality Control
The testing performed covers a wide range of categories, including but not limited to: Physical parameters, chemical parameters, HPTLC chromatographic fingerprinting, HPLC quantified active compounds, impurity profile, microbial profile, residual solvents, residual pesticides, heavy metals profile and aflatoxins. The stability of every ingredient is periodically monitored through the product's lifetime and elaborate documentation is maintained to ensure traceability at all levels.
Comprehensive Quality Assurance
KaraCalm is manufactured in ISO 22000 and GMP certified facilities. There are specific HACCP and HARPC procedures that are followed to analyze each step in the production process. This enables us to assess the risk of any potential biological, chemical or physical hazard and contamination, and if one is identified, we implement specific control measures to address the issue, ensuring the product is manufactured safely, and free of hazards and cross-contamination.
Control Samples & Stability Testing
Control samples for every single batch of KaraCalm is maintained to facilitate traceability and to ensure that it consistently meets our high-quality standards. Stability testing of the product, which includes real-time and accelerated stability testing, is also performed at specified time intervals. By performing these tests, it helps us determine the shelf life of our products and monitor the characteristics of the product over time to ensure continued efficacy and purity.
Command the Attention Your Brand Deserves!
Create a Product with KaraCalm™
CONTACT US TO LEARN HOW WE CAN WORK TOGETHER
Karallief® Inc.
Email: Sales@Karallief.com
Address: 75 Arlington St. Suite 500, Boston, MA 02116, USA
Scientific References
Gersappe V, Rajendran K, Layton K, Guan B, Gupta AK, Venkateshwarlu K. A randomized, double-blind, parallel and placebo-controlled clinical study to evaluate the efficacy and safety of KaraCalm™: a dietary supplement to support sleep and manage stress.Int JBasic Clin Pharmacol 2024;13:475-85.
KARALLIEF® INC. 75 Arlington St. Suite 500, Boston, MA 02116, USA | Sales@Karallief.com
© Copyright 2024
Privacy Policy
*These statements have not been evaluated by the Food and Drug Administration. This product is not intended to diagnose, treat, cure, or prevent any disease.
This website summarizes the results of a randomized, double-blind, parallel and placebo-controlled study. KaraCalm™ is not offered for sale to consumers. The information contained herein is only intended for use by manufacturers for product development purposes and should be reviewed by their legal counsel before the information is used on labels, in marketing or promotional materials or as support for any claim made in connection with a product that contains KaraCalm™.